Apricot, mirabelle, plum and prune
disease models
These 4 stonefruits and their relevants can be treated similar for the models we give for Taphrina, Shot Hole, Brown Rot, Scab and Rust.
The importance of some plant diseases varies from plant to plant. Partly this is due to different resistance or susceptibility or because of different growing climates.
Aphid Risk Model
Conditions:
- in the morning when sun raises and relative humidity decreases, optimum temperatures between 20°C and 32°C – good flight is indicated.
- If temperatures are not in the optimum range (too cold/hot) or it is too wet (leaf wetness) risk decreases.
- Output is the daily risk.
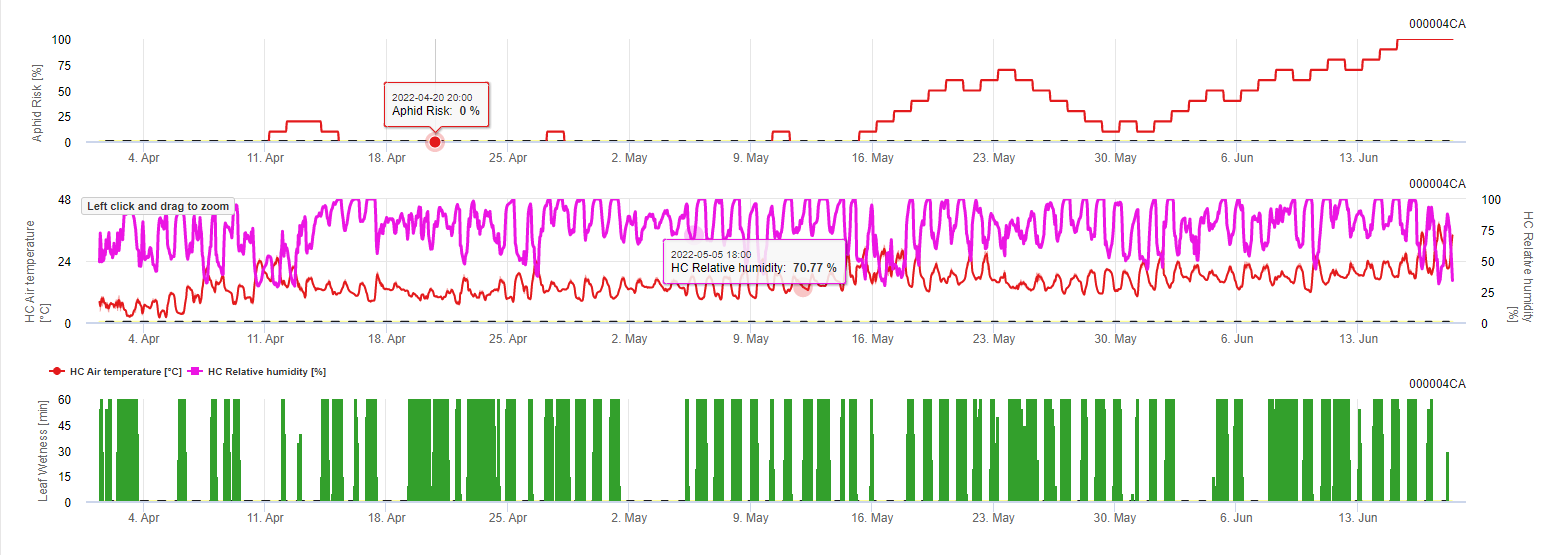
So optimum temperatures and falling relative humidities during the morning are indicating a good flight day. When it is wet during the night and temperatures are to low this is bad for propagation. The same when it is hot and moist during the day.
Xanthomonas arboricola
Symptoms
- leaves on peach: small, pale-green to yellow, circular or irregular spots. Spots enlarge, become darker to deep-purple, brown or black. The disease areas drop out, given a shot-hole appearance. A darf ring of disease tissue is left. Infected leaves turn yellow and drop off.
- leaves on plum fruit: symptoms may be different, large, sunken black lesions or only small pit- like lesions depending on the cultivar.
- twigs of peach: spring cankers occur on the overwintering twigs and on watersprouts before green shoots are produced; firstly small, water-soaked, dark blisters (1-10cm), sometimes girdle the twig, which causes the death of the top of the twig. Below the dead area (here are the bacteria present) a dark, so called “black trip”occurs.
- twigs of plum, apricot: cankers are perennial and continue to develop in twigs of 2 and 3 years old.
Xanthomonas arboricola pv. pruni is a bacterial disease and is listed as a quarantine pest in the EEP A2 list. Mainly species of Prunus spp. are attacked by the bacterium and particularly fruit crops as almonds, peaches, cherries, plums and apricots. X. arboricola occurs now worldwide, but was firstly found and described in North America (it is not realy clear if it spread from there or has naturally a wide distribution range).
Biology
Xanthomonas arboricola is a aerobic, gram-negative bacterium.
The bacterium overwinters in the intercellular spaces of the cortex, phloem and xylem parenchyma of the peach tree. On plum and apricot summer cankers are formed in one season, which develops the following spring and provides a source of inoculum. Also, plum buds and fallen leaves are an overwintering source for the bacterial disease.
In springtime the bacteria start to multiply and cause the epidermis to rupture- lesions are visible and are called spring canker. Inoculum from these cankers is disseminating by rain and wind and infect healthy plant tissues via stomata. On these leaves, lesions are developing, which exude bacteria and are called secondary infections.
Summer cankers develop in the green tissue of the shoot, but are sealed off by a periderm layer and dry out during summertime, which reduces the viability of the bacteria- therefore summer cankers in plum and peach are not of great importance as overwintering sites or initial infections the following season. In general, it is the late infections of shoots, occurring during rains just and during leaf fall in autumn which constitute the primary inoculum source for the following spring.
Modelling X. arboricola
Sensors: Air Temperature, relative Humidtiy, Leafwetness and Precipitation
in FieldClimate.com we have three models of X. arboricola, depending on the development stage of the plant/infection of different plant material (blossom, leaf and fruit infection) and a propagation model.
The bacterial disease is favored by warm, moderate seasons with temperatures of 10-28°C, light and frequently rainfalls with heavy winds and dews. Local dispersial is possible by rainsplash in orchards.
Severity classes depend on the inoculum (last year epidemiology, the susceptibility of the variety and the weather conditions).
1. Modelling Blossom Infection
Temperatures between 15°C and 30°C, leaf wetness is more than 0.
In the graph a weak infection of the blossom has been determined, a moderate and severe infection was not calculated (has to reach 100% on the line) because leaf wetness period was too short.
2. Modelling Fruit and Leaf Infection (additional precipitation is necessary to determine infections)
Temperatures between 15°C and 30°C, precipitation is more than 0, leaf wetness is more than 0, it is not night.
Also this model separates between weak, moderate and severe infections in dependence of the sum of rain. . A weak infection (temp. between 15 and 30°C, precipitation and leaf wetness) was determined on 16th of June because of the precipitation and the long leaf wetness period afterwards as well as the temperatures (10-17,5°C) during this time. A moderate infection (precipitation sum > 2mm) was not calculated (but nearly 98% of infection) as well as a severe infection (Precipitation sum > 5 mm) on that time.
3. Propagation Index
Temperatures between 15 and 30°C, leaf wetness more than 0 or relative humidity higher than 80%. Reset all 48hours. The graph shows 70% propagation on the 19th of July.
Monilla SPP. on plum and apricot
Brown rot, caused by Monilia spp. (Monilia laxa, Monilia fructigena and Monilia fructicola) belong to the most destructive diseases on stone fruits in Europe.
Symptoms
Symptoms of the brown rot disease are the blight of the blossom as well as the green tip of twigs due to the penetration of the pathogen into the open blossom through the stigma of pistils or anthers. This usually results in wilting of the whole part of an one-year-old twig. The leaves start to hang down, later they become brown and rigid, but usually do not fall down on the soil, they remain on the tree until the spring of the next year. Sometimes, especially under humid conditions droplets of gum are visible, which are symptoms of colonisation of the fungus as well as the established cankers.
The infected fruits are covered by putrefactive spots, from which warty sporodochia (hyphe) with conidia of the “summer” form appear. Additionally, in late autumn and winter, the fungus produces sporodochia of the “winter” form on infected twigs. With time, severely affected fruits become mummified. The mycelium growing in such mummies gradually aggregates into sclerotia. Such fruits remain on the tree during winter.
Temperature and wetness duration are important environmental factors, determining the infection incidence of em>M. laxa on blossoms. Monilia laxa is well adapted to the relatively low temperatures during spring and cause infections at temperatures as low as 5°C within a very short period of wetness duration. The infection of the active bloom trough the stima does not need very much leaf wetness. Leaf wetness is only needed for germination of the conidia. Therefore infection of the young fruit needs longer leaf wetness periods. To infect the young fruit an appressoria has to be formed and free moisture is needed to build up the pressure to form the infection peg to enter the epidermis cell. With maturity of the fruit small scars on the fruits allow an infection without infection peg again and the needed leaf wetness duration becomes shorter again.
The M. laxa model in fieldclimate.com calculates the risk of a Monilia infection in dependence of leaf wetness and air temperature.
Fieldclimate Modeling: It is probably that the time, needed for infection during bloom has to be shortened. Therefore the model is shorting infections down in the area of 2000 to 4800 degree hour above 5°C.
The graph shows the time of leaf wetness needed in dependence of the actual temperature.
Literature:
- Michailides, T., Luo, Y., Ma, Z., and Morgan, D.P. 2007. Brown Rot of Dried Plum in California: New Insights on an Old Disease. Online. APSnet Features. doi: 10.1094/APSnetFeature-2007-0307 (http://www.apsnet.org/publications/apsnetfeatures/Pages/BrownRot.aspx).
- Tian, S. P. and Bertolini, P. (1999), Effect of Temperature During Conidial Formation of Monilinia laxa on Conidial Size, Germination and Infection of Stored Nectarines. Journal of Phytopathology, 147: 635–641. doi: 10.1046/j.1439-0434.1999.00440.x
- Fourie and Holz 2006: Wound Infection of plum fruit by airborne conidia of Monilinia laxa
Shot hole
Shot hole is caused by the fungal pathogen Wilsonmyces carpophilus.
Most severe on apricots, but occurrs on all stone fruit. The fungal patogen infects the leaves, twigs and fruits.
Symptoms
Infected leaves show small brown spots with reddish margins (about 1 mm diameter), these spots expand to larger circular lesions (about 3mm diameter). These spots dry and fall out of the leaf, giving a shot hole appearance. The infected twigs show clear- cut brown margins with a necrotic center, which does not drop out, but ooze large amounts of gum. Further on lignification of infected twings is hindered and the lesions will grow into cankers. In severe cases premature defoliation of the tree may result.
Fruits show firstly small circular, deep purple spots. As the disease progresses, the symptoms differ according to fruit type. On apricots the spots become brown, raised and rough, giving the fruit a scabby surface. On peaches and nectarines the scabs develop into deep indentations.
Infected fruit have spots of gum and in severe cases cracks in the skin.
The shot hole fungus survives in infected buds. It is able to infect leaves, stems and fruits during cold, rainy weather periods in spring and autumn. Rain periods to infect healthy plant organs are needed.
The fungus is able to persist several years in the cankers or buds of infected twigs. Whenever conditions are favorable it may continue to grow, even during wintertime. In springtime, the conidia are splashed by rain to flowers and young leaves and infect them. In unfavourable periods (dry conditions) the conidia are still viable for several months. Rain is necessary for dispersal and humid conditions are needed for germination. The fungus is able to grow above 2°C.
When moisture is continuous for at least 24 hours and temperatures are above 2°C, conditions for infection are given. When temperatures are higher during the growing season, shorter periods of moisture are required for germination of the fungal pathogen; for example only 6 hours are needed at 25°C. Spores spread primarily by splashing water and can remain viable for several months under dry conditions. Under favorable conditions, spores can be produced from infected buds and stem lesions throughout the growing season. Most cultivars of peach, nectarine, apricot, and almond appear to be very susceptible. Cherry and plum are less susceptible and show only leaf and fruit symptoms when extended periods of moisture are present in late spring and early summer.
The FieldClimate Infection model for shot hole disease shows the infection progress lines for weak, moderate and severe infections. The model is similar to the modelling of apple scab. At the beginning of May a shot hole infection has been initialized by rain. The leaf wetness and the high relative humidity was lasting long enough to finish a weak and severe infection.
Powdery mildew
Powdery mildew is a common disease on many types of plants. Several powdery mildew fungi cause similar diseases on different plants (such as Podosphaera species on apple and stone fruits; Sphaerotheca species on berries and stone fruits; Erysiphe necator on grapevines). Powdery mildew fungi generally require moist conditions to release overwintering spores and for those spores to germinate and infect plant tissue. However, no moisture is needed for the fungus to establish itself and grow after infecting the plant. Powdery mildews normally favour warm, Mediterranean-type climates.
Powdery mildew can be recognized easily on most plants by the white to gray powdery mycelium and spore growth that forms on both sides of leaves, flowers, fruits and on shoots. On tree fruits a rough corky spot on the skin will develop where infection occurred.


All powdery mildew fungi require living plant tissue to grow. On deciduous perennial hosts such as grapevine, raspberry, and fruit trees, powdery mildew survives from one season to the next in infected buds or as fruiting bodies called chasmothecia, which reside on the bark of cordons, branches, and stems.
Most powdery mildew fungi grow as thin mycelium layer on the surface of the affected plant part. Spores, which are the primary means of dispersal, make up the bulk of the powdery growth and are produced in chains that can be seen with a hand lens. In contrast, spores of downy mildew grow on branched stalks that look like tiny trees. Also downy mildew colonies are gray instead of white and occur mostly on the lower leaf surface.
Powdery mildew spores are carried by wind to host plants. Although humidity requirements for germination vary, many powdery mildew species can germinate and infect in the absence of water. In fact, spores of some powdery mildew fungi are killed and germination and mycelial growth are inhibited by water on plant surfaces. Moderate temperatures and shade are generally the most favorable conditions for powdery mildew development, since spores and mycelium are sensitive to extreme heat and direct sunlight.
This fungus overwinters as mycelia inside the budscales, primary infection occurs as leaves emerge from these infected buds. Secondary infections occur when conidia produced by primary and subsequent secondary infections are blown or splashed by rain onto susceptible tissues. Fruit (before pit hardening) and succulent terminal growth are susceptible to infection.
The average minimum, optimum, and maximum temperatures for S. pannosa are about 5°, 24° and 24°C. Many more conidia are formed in dry air than in humid air at all temperatures (C.E. Yarwood, Soliman Sidky, Morris Cohen, Vincent Santilli; 1954)
Powdery mildew is common under similar relative humidity and temperatures as cherry powdery mildew.
Fieldclimate Model: Fungal disease is modeled by the factors temperature and duration of leaf wetness. For example on May 11th the leaf wetness period under moderate temperatures supported the development of the disease and a risk of 100% could be determined.
Literature:
- C.E. Yarwood, Soliman Sidky, Morris Cohen, Vincent Santilli (1954): Temperature relations of Powdery Mildews. HILGARDIA. A Journal of Agricultural Science Published by the California Agricultural Experiment Station. University of California. Volume 22/Number 17.
Taphrina leaf curl
Peach leaf curl (fungal pathogen: Taphrina deformans) is a fungus disease that can cause severe early defoliation and crop loss on nearly all peach and nectarine cultivars.
Symptoms
The most common and striking symptom of leaf curl occurs on the leaves (foliage). Infected leaves are severely deformed and often display a variety of colors (light green and yellow to shades of red and purple). The fungus causes the meristematic cells at leaf margins to proliferate quickly and randomly, which results in the leaves becoming variously wrinkled, puckered, and curled (photo 2). As these infected leaves mature, naked asci containing ascospores of the pathogen are produced on the surface giving them a dusty appearance, after which the leaves turn brown, shrivel, and drop from the tree.
Many infected fruits drop early and go unnoticed; those that remain may become crooked at the stem end like a small yellow squash, while others develop reddish to purple and have “wart-like” deformities on the surface.
Disease cycle
The pathogen occurs commonly almost wherever peaches are grown. The fungal pathogen overwinters as conidia (blastospores, “hyphal like” spores) in protected sites in the bark and around the buds. Primary infections occur during the early spring. Starting when the buds swell until the first leaves appear from the buds. Infections on young peach leaves occur at temperatures of 10°C to 21°C. Few infection occurs below 7°C. Infections appear mainly when rain wash the overwintered spores into the buds and cold temperatures lengthen the development time of the leaves (they are exposed for a long time to the pathogen before they are fully expanded and are able to resist the penetration of the fungus). If temperatures after bud swelling are warm and leaves develop quickly, infections rarely become established, even when spring rains occur. Wetness from rain (or other factors) for over 12,5 hours are needed for leaf infection but only when the temperature is below 61°F (is 16°C) during the wet period. Maximum infection occurs when trees are wet for 2 days or more, a frequent occurrence west of the Cascades. Although infected, symptoms may not appear if temperatures remain above 69°F (21°C). Fruit are susceptibel after petal fall until air temperature remains above 19°C. Rainfall of 0,5 inch and wetness of 24hours are needed for fruit infection.
The risk of a Taphrina defomans infection is calculated in FieldClimate on two ways:
- using temperature values (old model).
- using rain accumulation during the last consecutive hours and temperature during that time period. Further on this model includes also incubation time (time, when symptoms are seen in the field) using temperature below 19°C for calculations.
Both models are available in FieldClimate under “Taphrina Leaf Curl disease”.
Taphrina pruni infection model
As the species name suggests, Taphrina pruni (german: Narren- oder Taschenkrankheit) infects Prunus domesticus (Plum) and Prunus spinosus (Blackthorn or Sloe) fruits to form pocket plums. It also infects the shoots of Blackthorn to cause stunted or swollen distortions.
The fungal pathogen T. pruni does not produce fruit bodies. The spore germinates on the plant surface (forms an appressorium) and penetrates the flesh seeking refuge for nutrition. Infected fruits tend to become elongated, often more on one side than the other, which leads to to pocket-like shapes. The ascomycete fungi T. pruni produces its spores in tubes called asci (plural; singular = ascus). These asci penetrate through the surface of the fruit where the tip releases under pressure, shooting the spores out into the air.
Source: Microfungi on land plants: An Identification handbook. Richmond Publishing.
Symptoms
The gall is usually known as ‘pocket plum’, however alternatives are ‘starved plum’; ‘bladder bullace; and ‘mock plum’. The gall appears on the developing fruit, rendering it inedible and resulting in an elongated, flattened, hollow, stone-less gall of any colour from light green, through grey to light orange. The surface of the gall becomes corrugate and coated with the fungus, showing as a white bloom of ascospore producing hyphae. The totally inedible fruits shrivel and most fall. Some overwinter on the tree. Stems bearing deformed fruit may also thicken and grow with a deformation. The leaves are smaller and strap-like and shoots may be swollen, pale yellow and tinged with red. Cold and wet weather conditions promote the germination of spores, whilst warm and dry weather results in low infections.
In FieldClimate we model the risk of a Taphrina pruni infection in dependence of the actual temperature. The temperature has to be below 16°C.
Literature:
- REACTION OF SOME PLUM CULTIVARS TO NATURAL INFECTION WITH Taphrina pruni (Fuck.) Tul., Fusicladium Pruni DUCOMET AND Tranzschelia pruni-spinosae PERSOON DIETEL Mitre Ioana jr. 1) , V. Mitre1) , Erzsebet Buta1) , Ioana Mitre1) , Andreea Tripon1) , R. Sestras1)*
Stone fruit scab model
Stone fruit scab is induced by the plant pathogenic fungus Cladosporium carpophilum. The pathogen occurs on peaches, nectarines, apricots, and plums, while losses are generally greater on peaches than on the other fruits.
The disease affects twigs, leaves and fruits. The most serious damage results from fruit infections.
Symptoms
Fruit lesions start as small, round, greenish spots. These spots generally don’t appear until the fruit is half grown even though infection occurred earlier in the season (about six to seven weeks after petal fall). Older lesions are approximately 1/4 inch in diameter and develop a dusty or velvety green appearance. The numerous lesions typically are clustered near the stem end of the fruit (this site is exposed to the sun). Extensive spotting can result in fruit cracks, which serve as entrance points for several fruit rotting fungi. Fruits may also drop prematurely or could not be stored well.
Leaves could also be infected. Small, round and yellowish- green spots occurr on the undersite of the leaf. The plant tissue may dry und drop down, leaving shot- holes. In a rainy season the infected leaves usually drop early.
On twigs cankers begin as small, reddish lesions on current season’s growth. These cankers expand slowly and may not be visible until mid summer. The small cankers have irregular margins, but do not cause sunken areas on the bark.
On the twigs the mycelium (or conidia) hibernates in the form of dark-brown spherical cells. From overwintering mycelium, conidia are produced in the spring, and the latter are carried to the leaves and fruits by wind or by rain. The conditions which favor disease development are temperatures above 16°C for spore production, over 10°C (optimal 22°C to 27°C) for spore germination, and between2°C and 35°C for disease development. Germination and penetration into the plant tissue follows shortly. Inoculations and infections continue to take place until about one month before the fruit matures. As the fungus grows on the fruit the mycelium attaches itself closely to the surface between the hairs, forming a mat of short, plump cells which give rise to conidiophores and conidia. The flesh of the peach is not penetrated, but the close contact of the fungus with the outer cells allows absorption of nutrition from the fruit through the unbroken walls. Evidently there is some injury to the outer cells.
In FieldClimate the risk of a Caldosporium carpophilum infection is determined by wet conditions during spring and early summer after petal fall. The disease is usually more serious in low- lying, shady and moist areas with low air movement.
In FieldClimate we determine infections within a temperature range of 7 to 24°C, with a temperature optimum around 20°C.
The FieldClimate Modell calculates in dependence of leaf wetness duration and temperature a risk model of Cladosporium carpohilum.
Rust disease
Rust is caused by the fungal pathogen Tranzschelia discolor.
Symptoms
Common symptoms of the disease are twig cankers, leaf lesions and fruit lesions. Not all symptoms may develop in each growing season.
1. Twig cankers
Twig cankers are the first symptoms in the spring. This cankers develop after petal fall in spring during fruit development on one year old wood. Symptoms are seen as blisters and longitudinal splits in the bark.
The infection starts with water- soaked lesions, which swell and rupture the epidermal tissue of the twig. Cankers are usually found on the upper, redish side of the twig. Few days afterwards (depending on the temperature) the cankers mature and produce rusty brown powdery masses of spezialised spores (urediniospores). This urediniospores are spiny and sharply constricted at the base. At the end of the saison old cankers could be still observed, they may persist in the following season but no longer viable spores are produced.
2. Leaf lesions
Leaf lesions develop usually after cankers form in spring and may continue till autumn. Defoliation can occur when high numbers of infections are on single leaves. First infected leaves are in close to the twig cankers (infection source). Lesions develop as pale yellowish green spots visible on both leaf surfaces. The lesions become bright yellow and angular and with age necrotic in the center. On lower leaf surfaces numerous spore pustules (uredinia) can bee found. They become rusty brown due to the production of powdery masses of urediniospores. At the end of the season leaf lesions my turn tark brown to black and they produce two- celled teliospores. These leaf lesions are angular shaped, small size and rusty brown.
3. Fruit lesions
Fruit lesions develop during growing season after the symptoms of the leaves. Firstly brownish spots with green halos on mature, yellow fruits are seen. When fruit redden, the halos become greenish- yellow. Numerous infections develop on each fruit and these can lead to secondary infections by other fungal pathogens like Monilinia, Colletotrichum, Alternaria or Cladosporium.
Pathogen
The fungal pathogen attacks plants of the genus Prunus, including almond, apricot, cherry, peach, nectarine, plum and prune. The fungus can be seperated by special forms, based on the host where it is found. These forms are T. discolor f. sp. persicae on peach, T. discolor f. sp. dulcis on almond, T. discolor f. sp. domesticae on prune.
The fungus has multiple spore stages, which develop on two different hosts (alternate hosts). The only alternate host which is reported from California is Anemone coronaria (Ranunculaceae). The different spore stages are urediniospores, teliospores, basidiospores and aeciospores. Only urediospores and teliospores are found on Prunus sp.
The single celled, rusty brown urediniospores are produced on peach and can re-infect peaches. This secondary infection and additionally spore production and reinfection causes epidemic damages on peach. The teliospores, which develope late in the season on peach are not able to reinfect peach. After overwintering, the teliospores germinate and produce basidiospores that infect the alternate host Anemone coronaria.
Aeciospores that are produced on A. coronaria infect only Prunus spp. and the infection produces the first cycle of urediniospores in the spring. A. coronaria is rare in stonefruit yards and probably not the source of first infection in the yards.
The fungus probably overwinters as mycelium in infected fruit wood from the previous summer or fall. In spring these infections become the twig cankers and that are the source of primary inoculum each year. Urediniospores from twig cankers infect leaves, where more spores are produced in lesions and under favourable conditions the disease becomes epidemic.
Source: Adaskaveg JE, Soto-Estrada, A, Förster, H, Thompson, D, Hasey, J, Manji, BT, Teviotdale, B. (2000) Peach rust caused by Tranzschelia discolor in California. University of California. Agriculture and Natural Resources.
Conditions for an infection – Output in FieldClimate
Urediniospores are dispersed by wind and rainfall. They germinate over a wide temperature range from 5°C to 30°C with an optimal temperature range of 10-25°C. The viability of inoculum and wetness are major factors for determination infection periods.
Leaf and twig infections can occurr over a wide range of wetness period (12 to 36hours) and temperatures (15 to 25°C). Under controlled conditions the optimal wetness duration and temperature for infection was 18 to 36 hours at 15°C to 20°C. The incubation period after infection is 8 to 10 days, whereas the incubation period for twig symptoms is 4 to 6 weeks at 20°C.
Chilling portions
Chilling
Stone fruit trees develop their vegetative and fruiting buds in the summer and, as winter approaches, the already developed buds go dormant in response to both shorter day lengths and cooler temperatures. This dormancy or sleeping stage protects these buds from oncoming cold weather. Once buds have entered dormancy, they will be tolerant to temperatures much below freezing and will not grow in response to mid-winter warm spells. These buds remain dormant until they have accumulated sufficient chilling units (CU) of cold weather. When enough chilling accumulates, the buds are ready to grow in response to warm temperatures. As long as there have been enough CUs the flower and leaf buds develop normally. If the buds do not receive sufficient chilling temperatures during winter to completely release dormancy, trees will develop one or more of the physiological symptoms associated with insufficient chilling: 1) delayed foliation, 2) reduced fruit set and increased buttoning and, 3) reduced fruit quality.
Insufficient Chilling Symptoms
Delayed Foliation:
A classic symptom of insufficient chilling is delayed foliation. A tree may have a small tuft of leaves near the tips of the stems and be devoid of leaves for 12 to 20 inches below the tips. Lower buds will break eventually but full foliation is significantly delayed, fruit set is reduced, and the tree is weakened. Furthermore, heavy suckering from lower parts of the tree causes management problems, and normal development of next year’s fruit buds can be impaired.
Reduced Fruit Set and Buttoning:
Flowering, in response to insufficient chilling, often follows the pattern seen with leaf development. Bloom is delayed, extended, and due to abnormalities in pistil and pollen development, fruit set is reduced. In many peach cultivars, flowers drop before or around shuck split, but in others such as ‘Jersey Queen’ and ‘Harvester’, buttons form. Buttons result from flowers which apparently have set but never develop into full-size fruit. The fruit remains small and misshapen as they ripen. If you cut these fruit open, the seed is dead. Because buttoning is not apparent early in the season, growers can not thin off the abnormal fruit and the developing buttons serve as a food source and overwintering site for insects and diseases.
Reduced Fruit Quality:
The effects of insufficient chilling on fruit quality are probably the least discussed but appear to be very common especially in central and south Texas. The effects on leaf growth and fruit set are dramatic but the effects of insufficient chill on fruit quality are subtle and can occur when other symptoms do not. Insufficient chilling will cause many cultivars to have an enlarged tip and reduced firmness. Furthermore, fruit ground coloration may be greener than usual, possibly due to the fruit losing firmness before the ground color can fully change from green to yellow. The extent of these quality problems depends on the cultivar and the degree of chilling deficiency.
Models
There are various models used to calculate chilling, each one defining what a chilling unit is. The three most common models are the number of hours below 45 degrees F (7°C) model, the number of hours between 32 and 45 degrees F (2 and 7°C) model, and the Utah model. The first two models are simple and define a chilling unit as one hour below or between certain temperatures. The Utah method is more complex because it introduces the concept of relative chilling effectiveness and negative chilling accumulation (or chilling negation).
In fieldClimate.com we use the model for calculation of chill portions (CP). Chilling accumulations are calculated as chill portions, using a temperature range from 2 to 7°C. Calculations of chill proportions end after 96 hours of equal or more then >15°C ‘(it holds between7 and 15°C)
Calculations are based on the work of Erez A, Fishman S, Linsley- Noakes GC, Allan P (1990) The dynamic model for rest completion in peach buds. Acta Hortic 276: 165-174.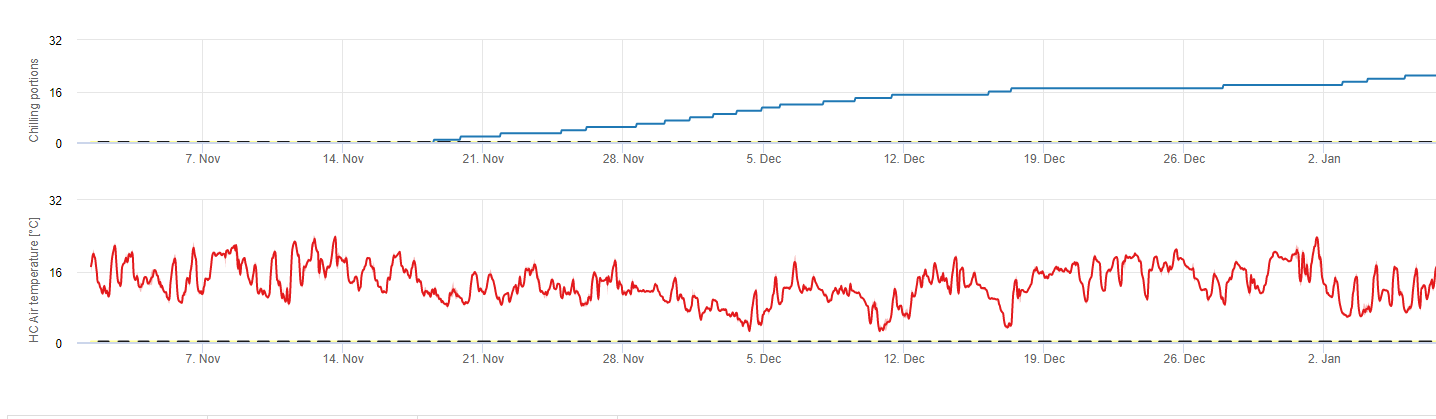

Rain accumulation
Intense rain will wash off the pesticides residuing on the leaves of vines or other plants. There has been a big improvement in the rain hardness of modern fungicides since 1980. Actually we can expect the most modern fungicide to resist up to 30 mm of rain if they had a chance to dry on the leaf. If the rain was starting immediately after the spray or trough the spraying the rain resistance might be widely reduced.
Old fashioned formulations of contact fungicides we have to expect a rain hardness of less than 12 mm. Like we were used to it during the 1970th. To wet the leaves in a vineyard it needs approximately 2 mm of rain. Therefore in this module, we only accumulate rains with are bigger than 2mm within one leaf wetness period. This means there might be in total 6 mm of rain during a single day, but this module is not accumulating any of it because the leaves has got dry again before it was raining 2 mm.
Rain is accumulated for 3, 5 and 7 days. Over a longer period plant growth is much more important for the effect of contact fungicides than rain resistance of the compounds.
In the following graph, you can see an example of accumulated rain for February and the first days of March for an iMETOS in a subtropical highland.
Recommended equipment
Check which sensor set is needed for monitoring this crop’s potential diseases.