Pear disease models
Rain accumulation
Intense rain will wash off the pesticide residue on the leaves of vines or other plants. There has been a big improvement of the rain hardness of modern fungicides since 1980. Actually we can expect the most modern fungicide to resist up to 30 mm of rain if they had a chance to dry on the leaf. If the rain was starting immediately after the spray or trough the spraying the rain resistance might be widely reduced.
Old fashioned formulations of contact fungicides we have to expect a rain hardness of less than 12 mm. Like we were used to it during the 1970th. To wet the leafes in a vineyard it needs approximately 2 mm or rain. Therefore in this module, we only accumulate rains with are bigger than 2mm within one leaf wetness period. This means there might be in total 6 mm of rain during a single day, but this module is not accumulating any of it because the leaves have got dry again before it was raining 2 mm.
Rain is accumulated for 3, 5 and 7 days. Over a longer period plant growth is much more important for the effect of contact fungicides than rain resistance of the compounds.
Fire blight
Source:P.W. Steiner, T. van der Zwet, and A. R. Biggs
Fire blight is a destructive bacterial disease of apples and pears that kills blossoms, shoots, limbs, and, sometimes, entire trees. The disease occurs in nearly all moderate to warm apple growing areas worldwide. Although outbreaks are typically very erratic, causing severe losses in some orchards in some years and little or no significant damage in others. This erratic occurrence is attributed to differences in the availability of overwintering inoculum, the specific requirements governing infection, variations in specific local weather conditions, and the stage of development of the cultivars available. The destructive potential and sporadic nature of fire blight, along with the fact that epidemics often develop in several different phases, make this disease difficult and costly to control.
Symptoms
Overwintering cankers harbouring the fire blight pathogen are often clearly visible on trunks and large limbs as slightly to deeply depressed areas of discolored bark, which are sometimes cracked about the margins. The largest number of cankers, however, are much smaller and not so easily distinguished. These occur on small limbs where blossom or shoot infections occurred the previous year and often around cuts made to remove blighted limbs. Since many of these cankers are established later in the season, they are not often strongly depressed and seldom show bark cracks at their margins. Also, they are often quite small, extending less than 2.5 cm, with reddish to purple bark that may be covered with tiny black fungus fruiting bodies (most notably Botryosphaeria obtusa, the black rot pathogen of apple).
Blossom blight symptoms most often appear within one to two weeks after bloom and usually involve the entire blossom cluster, which wilts and dies, turning brown on apple and quite black on pear. When weather is favorable for pathogen development, globules of bacterial ooze can be seen on the blossoms. The spur bearing the blossom cluster also dies and the infection may spread into and kill portions of the supporting limb. The tips of young infected shoots wilt, forming a very typical “shepherd’s crook” symptom. Older shoots that become infected after they develop about 20 leaves may not show this curling symptom at the tip. As the infection spreads down the shoot axis, the leaves first show dark streaks in the mid veins, then wilt and turn brown, remaining tightly attached to the shoot throughout the season. As with blossom infections, the pathogen often invades and kills a portion of the limb supporting the infected shoot. The first symptom on water sprouts and shoots that are invaded systemically from nearby active cankers is the development of a yellow to orange discoloration of the shoot tip before wilting occurs. In addition, the petioles and mid veins of the basal leaves on such sprouts usually become necrotic before those at the shoot tip.
Depending on the cultivar and its stage of development at the time infection occurs, a single blossom or shoot infection can result in the death of an entire limb, and where the central leader or trunk of the tree is invaded, a major portion of the tree can be killed in just one season. In general, infections of any type that occur between petal fall and terminal bud set usually lead to the greatest limb and tree loss. In addition, heavily structured trees tend to suffer less severe limb loss than those trained to weaker systems for high productivity. Where highly susceptible apple rootstocks (M.26, M.9) become infected, much of the scion trunk and major limbs above the graft union very typically remain symptomless, while a distinct dark brown canker develops around the rootstock. As this rootstock canker girdles the tree, the upper portion shows symptoms of general decline (poor foliage color, weak growth) by mid to late season. In some instances, the foliage of trees affected by rootstock blight develop early fall red color in late August to early September, not unlike that often associated with collar rot disease caused by a soil borne fungus. Some trees with rootstock infections may not show decline symptoms until the following spring, at which time cankers can be seen extending upward into the lower trunk.
Disease Cycle
The bacterial pathogen causing fire blight overwinters almost exclusively in cankers on limbs infected the previous season. The largest number of cankers and, hence, those most important in contributing inoculum, occur on limbs smaller than 38 mm in diameter, especially around cuts made the previous year to remove blighted limbs. During the early spring, in response to warmer temperatures and rapid bud development, the bacteria at canker margins begin multiplying rapidly and produce a thick yellowish to white ooze that is elaborated onto the bark surface up to several weeks before the bloom period. Many insect species (predominantly flies) are attracted to the ooze, and subsequently, disperse the bacteria throughout the orchard. Once the first few open blossoms are colonized by the bacteria, pollinating insects rapidly move the pathogen to other flowers, initiating more blossom blight. These colonized flowers are subject to infection within minutes after any wetting event caused by rain or heavy dew when the average daily temperatures are equal to or greater than 16 °C while the flower petals are intact (flower receptacles and young fruits are resistant after petal fall). Once blossom infections occur, early symptoms can be expected with the accumulation of at least 57 degree days (DD) greater than 13 °C which, depending upon daily temperatures, may require 5 to 30 calendar days.
With the appearance of blossom blight symptoms, the number, and distribution of inoculum sources in the orchard increase greatly. Inoculum from these sources is further spread by wind, rain, and many casual insect visitors to young shoot tips, increasing the likelihood of an outbreak of shoot blight. Recent research conducted in Pennsylvania indicates that aphid feeding does not contribute to shoot blight. More research is needed to determine whether or not leafhopper’s play a role in the incidence of shoot blight. Most shoot tip infections occur between the time that the shoots have about nine to ten leaves and terminal bud set, when sources of inoculum and insect vectors are available, and daily temperatures average 16 °C or more.
In years when blossom infections do not occur, the primary sources of inoculum for the shoot blight phase are the overwintering cankers and, in particular, young water sprouts near these cankers, which become infected as the bacteria move into them systemically from the canker margins. Such systemic shoot infections, called canker blight, are apparently initiated about 111 DD greater than 13 °C after green tip, although visible symptoms may not be apparent until the accumulation of at least 157 DD greater than 13 °C after green tip. In the absence of blossom infections, the development of shoot blight infections is often localized around areas with overwintering cankers.
Although mature shoot and limb tissues are generally resistant to infection by E. amylovora, injuries caused by hail, late frosts of -2 °C or lower, and high winds that damage the foliage can create a trauma blight situation in which the normal defense mechanisms in mature tissues are breached and infections occur. Instances of trauma blight are known to occur even on normally resistant cultivars like ‘Delicious’.
Rootstock blight, yet another phase of fire blight, has been recognized recently and is associated primarily with the highly susceptible M.26, M.9 and Mark rootstocks. On these trees, just a few blossom or shoot infections on the scion cultivar can supply bacteria that then move systemically into the rootstock where a canker often, but not always, develops and eventually girdles the tree. Trees affected by rootstock blight generally show symptoms of decline and early death by mid to late season, but may not be apparent until the following spring.
(c) Dr. Heinrich Denzer, Pessl Instruments GmbH, Weiz, 2007
Model Cougar Blight for Pears
Symptoms
The model requires the user to recognize specific and ever-changing local events and aspects of their orchard that may increase or decrease fire blight risk relative to other orchards in the region. The model requires the user to assume there is a risk of fire blight infection whenever blossoms are present on the trees, especially during the petal fall and “post-bloom” period, when scattered blossoms may remain on many apple and pear varieties. The model user is asked to carefully assess the situation on their specific site and to initiate control measures if blossoms are present, risk levels are “High” or “Extreme,” and blossom wetting is likely to occur sometime during the next 24 hours.
Model Structure
Temperatures and Wetness: The key Fire Blight process that must be modeled is the potential for bacterial growth on the stigmas of apple and pear flowers. This growth is temperature dependent, so dependable prediction of infection risk requires the use of a measurement method that most accurately reflects the growth of Erwinia amylovora colonies. The main disagreement among models is how this should be done.
Fire Blight Model Output in FieldClimate
The Cougarblight model estimates the bacterial growth rate with degree hours based on a specific growth rate curve. This growth curve is based on the growth rate of E. amylovora bacteria in laboratory tests. The degree hour values are accumulated each hour of the day that temperatures are over 15 °C. The hourly values increase as temperatures rise from 15 °C to 29°C, decline at higher temperatures, and reach zero for an hour with temperatures over 40°C.
Model Blossom Blight for Pears
- Flower must be open with stigmas and petals intact, stigmas have to be exposed for colonization, flowers in petal fall are resistant;
- Accumulation of at least 110 °C hours > 18.3°C within the last 66 °C days > 4.4 °C defines the epiphytic infection potential for the oldest open and hence most colonized flower in the orchard
- A wetting event occurring as dew or 0.2 mm of rain or 2.5 mm of rain the previos day allows movement of bacteria from colonized stigmas to the nectarthodes
- An average daily temperature of >= 15.6 °C: This may influence the rate at which the bacteria migrate into the nectarthodes as well as the multiplication of bacteria needed to establish infections.
When all four of these minimum requirements are met in the sequence shown, infections occur and the first early symptoms of blossom blight can be expected to appear with the accumulation of an additional 57 °C days > 12.7 °C. This can be 5 to 30 days after infection. When the orchard conditions are less than these minimum requirements, few or no symptoms occur and no significant epidemic develops. (STEINER P.W. 1996)
Graphical Presentation Fire Blight on Pears
In FieldClimate, the two fireblight models are displayed in one graph. The Cougar Blight model is named Fire Blight DIV and the Maryblight model is named Blossomblight. To interpret the Cougar Blight results the graph is underlaid in 5 different colors. The distribution of this colors is made on the base of the settings on the first blight history of the orchard. The 5 colors are indicating the risk class for the DIV values.
The opportunity of a Blossom Infection is indicated by a bar ranging from 0 – 1 (conditions are fulfilled or not) in the same graph. Settings about the history of the orchard are not integrated into this model. Each time a bar with blossom infection is calculated in FieldClimate.com is an infection with fireblight!
Practical Use Fire Blight on Pears
Aim of the Fire Blight Models is it to assess the probability of infections by Erwinia amyloflora in the orchard.
The Mary Blight model which is evaluating for blossom blight is very well indicating infection situations of high economic impact. For this reason it is quite frequently used to indicate the use of antibiotics against this pathogen.
Cougar Blight is giving information about the risk of fire blight infections do to the overall propagation possibilities of the pathogen. Its weighting done by the history of an orchard is very helpful to indicate to us how carefully we have to check the orchards for fire blight symptoms even in situations where Mary Blight will not indicate an infection.
(c) Dr. Heinrich Denzer, Pessl Instruments GmbH, Weiz, 2008
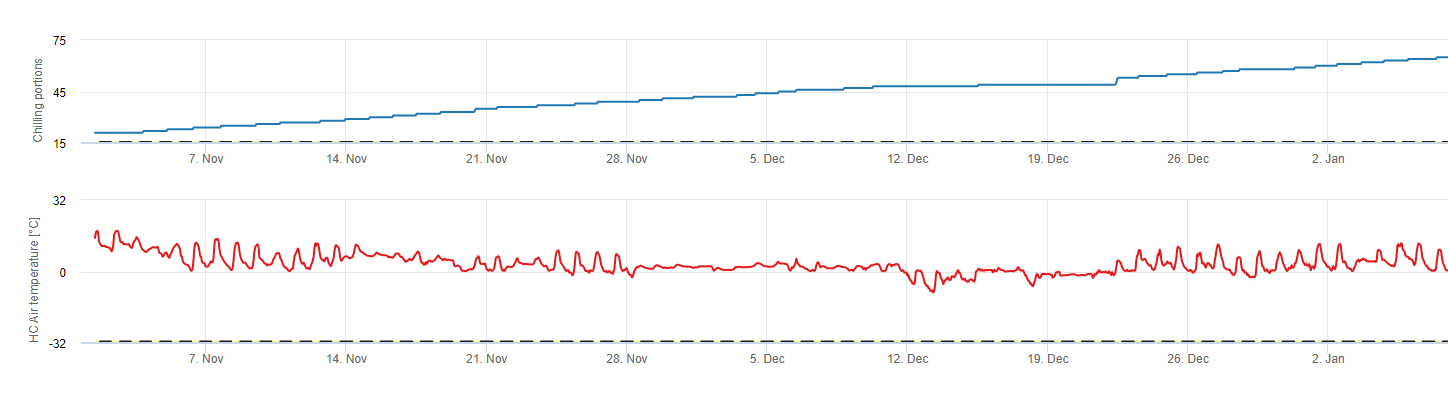
Chilling portions
Chilling
Stone fruit trees develop their vegetative and fruiting buds in the summer and, as winter approaches, the already developed buds go dormant in response to both shorter day lengths and cooler temperatures. This dormancy or sleeping stage protects these buds from oncoming cold weather. Once buds have entered dormancy, they will be tolerant to temperatures much below freezing and will not grow in response to mid-winter warm spells. These buds remain dormant until they have accumulated sufficient chilling units (CU) of cold weather. When enough chilling accumulates, the buds are ready to grow in response to warm temperatures. As long as there have been enough CUs the flower and leaf buds develop normally. If the buds do not receive sufficient chilling temperatures during winter to completely release dormancy, trees will develop one or more of the physiological symptoms associated with insufficient chilling: 1) delayed foliation, 2) reduced fruit set and increased buttoning and, 3) reduced fruit quality.
Insufficient Chilling Symptoms
Delayed Foliation:
A classic symptom of insufficient chilling is delayed foliation. A tree may have a small tuft of leaves near the tips of the stems and be devoid of leaves for 12 to 20 inches below the tips. Lower buds will break eventually but full foliation is significantly delayed, fruit set is reduced, and the tree is weakened. Furthermore, heavy suckering from lower parts of the tree causes management problems, and normal development of next year’s fruit buds can be impaired.
Reduced Fruit Set and Buttoning:
Flowering, in response to insufficient chilling, often follows the pattern seen with leaf development. Bloom is delayed, extended, and due to abnormalities in pistil and pollen development, fruit set is reduced. In many peach cultivars, flowers drop before or around shuck split, but in others such as ‘Jersey Queen’ and ‘Harvester’, buttons form. Buttons result from flowers which apparently have set but never develop into full-size fruit. The fruit remains small and misshapen as they ripen. If you cut these fruit open, the seed is dead. Because buttoning is not apparent early in the season, growers can not thin off the abnormal fruit and the developing buttons serve as a food source and overwintering site for insects and diseases.
Reduced Fruit Quality:
The effects of insufficient chilling on fruit quality are probably the least discussed but appear to be very common especially in central and south Texas. The effects on leaf growth and fruit set are dramatic but the effects of insufficient chill on fruit quality are subtle and can occur when other symptoms do not. Insufficient chilling will cause many cultivars to have an enlarged tip and reduced firmness. Furthermore, fruit ground coloration may be greener than usual, possibly due to the fruit losing firmness before the ground color can fully change from green to yellow. The extent of these quality problems depends on the cultivar and the degree of chilling deficiency.
Models
There are various models used to calculate chilling, each one defining what a chilling unit is. The three most common models are the number of hours below 45 degrees F (7°C) model, the number of hours between 32 and 45 degrees F (2 and 7°C) model, and the Utah model. The first two models are simple and define a chilling unit as one hour below or between certain temperatures. The Utah method is more complex because it introduces the concept of relative chilling effectiveness and negative chilling accumulation (or chilling negation).
In FieldClimate we use the model for calculation of chill portions (CP). Chilling accumulations are calculated as chill portions, using a temperature range from 2 to 7°C. Calculations of chill proportions end after 96 hours of equal or more then >15°C ‘(it holds between7 and 15°C)
Calculations are based on the work of Erez A, Fishman S, Linsley- Noakes GC, Allan P (1990) The dynamic model for rest completion in peach buds. Acta Hortic 276: 165-174.
Pear scab
Source: J.W. Travis, J.L. Rytter, and K.S. Yoder
Introduction
Pear scab is an economically important disease throughout the world and can cause serious losses on susceptible cultivars. The disease is more of a problem in European countries than in North America and is especially of major concern in Japan. Sometimes called the black spot, pear scab resembles apple scab (Venturia inaequalis) in nearly all respects, and is caused by the closely related fungus, V. pirina. Pear cultivars differ in susceptibility to scab; however, cultivars resistant in one region of the country may not be resistant in another region.
Symptoms
Symptoms of pear scab are very similar to apple scab. Lesions on leaves and petioles begin as round, brownish spots that eventually become velvety in appearance. Within these lesions, conidia are produced. Later in the season, small spots can be observed on the lower surface of the leaves. These are usually the result of late spring or early summer infections. Leaf infection of pear is not as common as apple scab on apple leaves.
Disease Cycle
Scab lesions on fruit occur on the calyx end and eventually on the sides of the fruit. As these lesions enlarge, they become dark brown and form large black areas as they coalesce. Lesions on immature fruit are small, circular, velvety spots. Darker, pinpoint spots develop as the fruit matures. Infected fruit often becomes irregular in shape. Unlike apple scab, twig infections are common with pear scab. Early in the growing season, lesions on young shoots appear as brown, velvety spots. Later, these lesions become corky, canker-like areas. The following spring, pustules will develop within these overwintered lesions. These pustules produce spores (conidia) that perpetuate the spread of the disease. The fungus overwinters in leaves on the ground and also as mycelium in infected twigs. Infection of pear foliage and fruit occurs under conditions similar to those required for infection of apple by the apple scab fungus. Ascospores are the major source of primary inoculum. Infection occurs in the spring around the green-tip stage of flower bud development. Ascospores in the overwintered leaves are released as the result of rain and are carried by air currents to young leaves and fruit. Ascospores continue to mature over a six to eight week period. Conidia are the source of secondary inoculum and are produced in either the primary lesions initiated by Ascospores or within pustules on infected twigs. Many secondary cycles may occur over a growing season. The length of the wetting period and temperature required for infection depend on the number of hours of continuous wetness and the temperature during this wetting period===. The Mills chart for determining apple scab infection periods along with a leaf wetness recorder or hygrothermograph can provide the information for determining the infection periods for pear scab. Scab lesions may develop in as few as eight days after infection on young leaves and in as many as two months on older leaves. Fruits are also more susceptible when young; however, mature fruit can be infected if the length of the wetting period is sufficiently long.
Monitoring
No monitoring required by growers during the dormant period. Consult with regional Cooperative Extension Service personnel to determine the onset of Ascospore maturity. An awareness of the scab inoculum situation in adjacent abandoned or commercial orchards may influence early-season scab control decisions. During the pre-bloom period and continuing through fruit set, for both fresh and processing fruit, determine pear scab infection periods by observing the duration of leaf wetness and average temperatures during the wet period.
The Venturia pirina Infection Model designed by Spotts, R. A., and Cervantes, L. A. 1991
Environmental Input variables: Temperature, wetness duration.
Model description: Spotts and Cervantes present data from a controlled environment experiment with pear seedlings as well as in-field limb bagging experiments on the effects of temperature and wetness duration on conidial infections of pear seedlings, leaves, and fruit. They have not evaluated Ascospore infection conditions but suggest that they should be quite similar to conidial infection conditions and therefore their model can be used to predict primary infection by Ascospores.
Action threshold: Model developers observed that the minimum wetness duration required for foliage infection by conidia all fell between the values required for “light” and “moderate” infection of apple by V. inequalis. Ascospores according to the Mills table. Therefore when using the Mills table for pear scab Ascospore or conidia infection, the authors recommend the use of hours of wetness for “light” infection to be more conservative.
(c) Dr. Heinrich Denzer, Pessl Instruments GmbH, Weiz, 2009
Fabraea leaf spot
Leaf spot, caused by the fungus Fabrea maculatum, is a widespread and destructive disease of red tip (Photinia fraseri), loquat (Eriobotrya japonica), India hawthorn (Rhaphiolepis indica), some pear cultivars (Pyrus sp.) and several other members of the rose family. This disease is most damaging to plants in the landscape and nurseries during periods of cool, wet weather and when active growth is occurring.
Symptoms
Tiny, circular, bright red spots on both the upper and lower surfaces of young expanding leaves are the first symptoms of Entomosporium leaf spot. Numerous small spots may coalesce into large maroon blotches on heavily diseased leaves. Leaf spots on mature leaves have ash brown to light grey centers with a distinctive deep red to maroon border. Tiny black specks, spore producing bodies of the fungus, can often be observed in the center of each leaf spot. Spots similar to those on the leaves can develop on leaf petioles and tender stem growth during prolonged periods of cool, wet weather.
Low levels of leaf spot usually cause little more than cosmetic damage but maintain a source of spores for future infections. Severe infections, however, often result in early and heavy leaf drop. Heavy leaf drop severely reduces the landscape value of red tip and can cause plant death. Some cultivars of India hawthorn are as severely affected as red tip.
Disease Cycle
Spots on the leaves and young shoots are important in the survival of the Entomosporium leaf spot fungus. Fallen, diseases leaves are less important sources of the fungus. Masses of spores are released during periods of wet weather from the fungal spore producing structures in the center of the spots from late winter through much of the year except during the hot periods of summer. These spores are spread to healthy foliage by a combination of splashing water and wind. New leaf spot symptoms appear within 10-14 days after a wet infection period.nfection Model: The four-celled conidia,with a distinctive insect-like appearance, are spread mainly from overwintering leaf litter, and some from twig cankers, by splashing water from rains or overhead irrigation. Wetting periods for infection may vary from 8 to 12 hours at temperatures of 10°C – 25°C. Lesions begin to appear about 7 days after the beginning of an infection period. The disease may advance rapidly in late summer as wind and rain distribute the conidia throughout the tree. Susceptibility of leaves and fruit to infection does not decrease with maturity. Nearly all pears of European descent are susceptible to this leaf spot. The model is started when leaf wetness starts with rain. It stops when leaf wetness is disrupted for longer than 1 hour.
(c) Dr. Heinrich Denzer, Pessl Instruments GmbH, Weiz, 2008
Brown spot
Brown spot on pear are caused by the pathogenic fungus Stemphylium vesicarium, which also causes disease on garlic, leek, onion and asparagus. On pear, the fungus infects leaves, fruits and to a lesser extent twigs. The resulting necrosis and fruit rot is caused by fungal penetration of stomata and lenticels and the production of chemical compounds which forces the host to kill the cells in the infected area, causing the brown spots. Brown spots causes severe damage especially in southern Europe. However, the disease was also found in the Additionally, the predominant pear cultivar in the Netherlands and Belgium, is very susceptible (Montesinos et al., 1995a).
The influence of temperature and wetness duration on conidial infection by S. vesicarium on pear has been studied previously (Montesinos et al., 1995b). The findings there led to the development of a brown spot forecasting system (Llorente et al., 2000). Climatical conditions in Europe are differing form South to North. In Southern Europe we can expect thunderstorms even during warm periods, whereas in Northern Europe cool raining periods lasting for several hours can occur even during mid summer. It is obvious that the forecasting system should be revalidated or even adapted for use under different climatical conditions.
The moist periods leading to Stemphylium versicarium infections on pear have to be very long following this model. The model goes back to the work carried out by Llorente, I., Vilardell, P., Moragrega, C. and Montesinos, E. and the adoption to electronic weather stations done by A. Boshuizen, P.F. de Jong and B. Heijne from Netherlands. This moist periods can be disrupted. The length of disruption depends on the relative humidity or the vapor pressure deficit.
In the calculation of the model of FieldClimatethe disruption can last for ever as long as the relative humidity is higher or equal to 75%. With a relative humidity in between 65% and 74% the disruption of the moist period can last for 12 hours. If the relative humidity is in between 55% and 64% the disruption can last for 9 hours. If relative humidity is in between 45% and 54% the disruption can last for 8 hours. If relative humidity is in between 35% and 44% the disruption can last for 6 hours. If relative humidity is below 34% the disruption can last for 4 hours.
The curves showing the progress of light, moderate and severe infections can be used as action thresholds for orchards with a differing disease history. In orchards with more than 1% disease incidence on fruit chemical control of the disease should be done on all light infections. In orchards with less than 1% of disease incidence on fruits chemical control should be done on all completed moderate infections. And in orchards which had up to now no occurrence of Stemphylium control methods should be started if severe infections will reach 100%.
Conditions:
Temperature: 8°C – 38°C
Leaf wetness > 0 or rel. humidity >90%
Factor: 600; max: 60000 (100% infection).
(c) Dr. Heinrich Denzer, Pessl Instruments GmbH, Weiz, 2009
Recommended equipment
Check which sensor set is needed for monitoring this crop’s potential diseases.